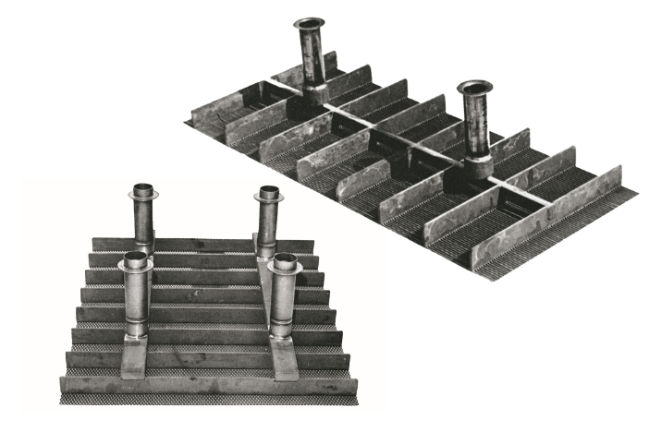
Anodes- electrodes of the new DSA®s
In the 1970s, one of the most significant advancements in industrial chemistry was the development of the DSA® electrode technology. These metallic insoluble anodes significantly improved the energy efficiency of chlor-alkali processes. Specifically, DSA electrodes consist of a titanium base coated with a thin layer of mixed metal oxides (MMOs) that act as electrocatalysts for a wide variety of electrochemical processes, including the production of chlorine at the anode and formation of sodium hydroxide and hydrogen at the cathode of an electrochemical cell.
Before the breakthrough of DSA electrodes, carbon materials like graphite and amorphous carbon were used for the anodes, but they had several drawbacks. In fact, the theoretical voltage for the reactions for the chlor-alkali process based on thermodynamics (i.e., the reversible potentials of the anode and cathode) is slightly above 2.1 V. However, before the introduction of DSA, the actual voltage was nearly 5 V due to slow reaction kinetics, mass-transport limitations, and Ohmic losses, leading to high electricity consumption and increased production costs. Additionally, carbon anodes were highly susceptible to corrosion (as displayed in equation 1), causing additional increases in the voltage at the reactor over time and frequent interruption of the production process due to the necessary replacement of the anodes.
C + 2H2O → CO2 + 4H+ + 4e- (1)

