Electrodeposited copper foil has become a hot topic in the recent years due to the energy transition, sustainability, and global supply chain geopolitical issues. Moreover, ED copper foil is a key material in our modern digital world, it is used in all electronic devices from computer and phones to the simplest electronic equipment. Therefore, ED copper foil is considered a key and strategical material for our society future.
In this blog article, we will see the ED copper foil manufacturing key processes. Starting with the most important part, the electrodeposition process with the cathode Titanium drums and the anodes, followed by the surface treatment with their anodes.
Electrodeposited (ED) Copper Foil Manufacturing Key Processes
Electrodeposited Copper Foil Manufacturing Process: Electrodeposition step
Why is the electrolytic process important in copper foil manufacturing?
The electrolysis process is the process of reducing and depositing dissolved copper ions with an electric current to form a thin metal layer like metal foil on the surface of the cathode titanium drum.
The quality of electrolytic copper foil varies greatly depending on the raw material, current density, and additives used for electrolysis. The surface roughness value of the electrolytic copper foil also changes. Why is the roughness of the electrolytic copper foil important? The smoothness and roughness of the electrodeposited copper foil surface have a significant effect (conductor loss) on the adhesion to various substrate materials and the current flowing through the copper foil on printed circuit boards.
In other words, the smoother the surface of the electrodeposited copper foil, the better the current flow (less conductor loss) and the smoother flow of signals in the high frequency band (reduction of transmission loss).

1mm detachable anodes with anode structure
Electrolytic copper foil electrolysis process requires two types of electrodes: cathode and anode. Electrolyzers generally consist of a cathode titanium drum and an anode, DSA® .
Why are the cathode (titanium drum) and the anode (anode structure) important in the electrolytic process of electrolytic copper foil?
Cathode (Titanium drum)
Through an electrolysis process, copper metal foil is reduced and deposited on the surface of the cathode titanium drum. Since this cathode is curved, it has a drum-like structure. The thickness of the copper foil can be controlled by the rotation speed and current density of the drum. Furthermore, the surface condition of the cathode titanium drum affects the roughness of the copper foil surface. As a result, both shiny and matte copper foils facing the drum can be achieved.
Anodes and Anode Structures
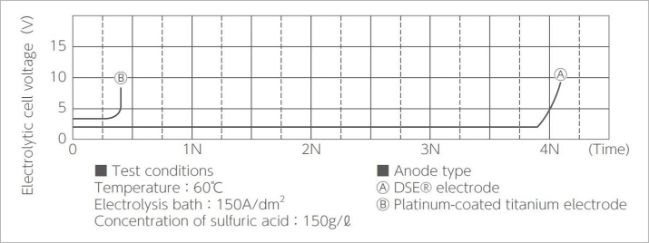
Figure 3 – example of accelerated lifetime test for DSA® anode
The anode also plays an important role in the copper electrolytic process. In optimizing the manufacturing process, the anode must be shaped to match the structure of the cathode titanium drum. Therefore, a radial anode should theoretically be the best choice. However, considering economic aspects such as cost and maintenance, it can be said that adopting a removable anode is the most suitable anode structure (see image above).
In fact, De Nora produces anodes for such an electrolytic copper manufacturing process. DSA® anodes have multiple advantages over other anodes such as: long life of anode and energy savings.
The lifetime of DSA® anodes is shown in Figure 3.
The results show that the DSA® anode has a longer life than the platinum-coated titanium electrode. The base material of the DSA® electrode hardly deforms or wears out due to electrolysis.
Figure 4 – potential of DSA® anode in sulfuric acid
Figure 4 shows the potential of the anode in sulfuric acid.
It can be seen that the DSA® anode contributes to saving energy and reducing energy costs.
- All anodes and anode structure designs (removable anode, radial, segmented anode), adopt advanced or patented technology.
- De Nora anodes can be used under a variety of working conditions and can produce foils of all qualities and thicknesses (including ultra-thin foils).
Electrodeposited Copper Foil Manufacturing Process: Surface treatment step
Why is surface treatment an important process in the production of copper foil for printed circuit boards?
Different surface treatments are used in the manufacturing process of electrolytic copper foil depending on the purpose. First, the surface of the copper foil itself is washed to deposit copper nodules. Doing so will change the roughness of the foil.
After that, a corrosion prevention process is performed to prevent oxidation of the surface of the copper foil (prevent copper oxide).
This prevents the copper foil's conductive performance from degrading over time. As a finishing touch, we protect the copper foil from chemicals and heat. This makes copper foil resistant to a wide range of chemicals and heat, as well as some physical influences.
There are various types of surface treatment depending on the application, and in order to impart such properties to the copper foil, each anode is customized to such needs.
In fact, De Nora manufactures anodes for the electrodeposited copper foil surface treatment manufacturing process. DSA® anodes offer several advantages over other anodes, including:
- Energy saving
- Long life of anode
- Specific properties tailored to this application
There are two important processes in the copper foil manufacturing process: electrolysis and surface treatment. Anodes play an important role in each process and without them the manufacturing process would not be possible efficiently. That's why De Nora aims to provide the best solution for each customer's needs and help them achieve their manufacturing goals.
[Biography] Hideto Shimizu
Global Product Manager
General Manager of New Business Promotion Department
Employed at De Nora Permelec Co., Ltd. for over 25 years.
He has been a sales manager in China for 5 years.
He has extensive practical experience in design, production technology, research and development, and sales.